This article was published as a part of the Data Science Blogathon.
Introduction
Leading biopharmaceutical industries, start-ups, and scientists are integrating Machine Learning (ML) and Artificial Intelligence Learning (AIL) into R&D to analyze extensive large data & data sets, identify patterns, and generate algorithms to explain them. AIL is increasingly capable of predictive analytics and problem solving, discovering and inventing innovations without human input. AI has the potential to transform R&D scale processes into the biopharmaceutical industrial scale and also ensure patient safety.
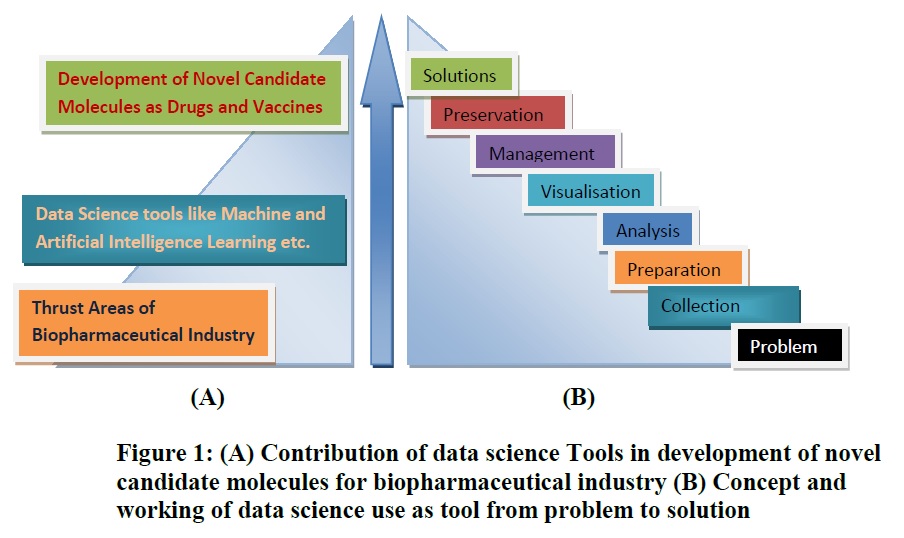
ML is directly related to finding patterns in the data and developing different data sets after employing the various techniques as information. The information will consider for improvement in the manufacturing process of drugs and vaccines in the biopharmaceutical industry and also under consideration as favorable input by scientists for the production, testing, and safety aspect of novel vaccines and drug molecules. This article will play a tremendous role in enhancing the knowledge of academicians and industry professionals. It could be helpful in the development of innovative and novel molecules of vaccines and drugs after applying ML and AI as data science tools in thrust areas of the biopharmaceutical as described in figure 1.
Biopharmaceutical Professionals
Biopharmaceutics is the study that shows how the drug absorption rate is affected by different factors like the physical and chemical properties of a drug, drug dosages, drug form, and drug route of administration. The study deals with drug stability, the liberation of the API from the dosage form, the conversion rate of the drug into excretable form, and the absorption of the drug into the systemic circulation. Biopharmaceutical professionals belong to the following working area in academic, R&D, and industries as shown in figure 2, and the detail of the working areas as shown in table 1.
Table 1: Working Area of Biopharmaceutical professionals
| S.No. |
Working Area |
Description |
|
| 1 | Pharmaceutics | Pharmaceutics is the core branch of pharmacy. It deals with the science of developing a new chemical entity (NCE) or old drugs into a medication that can be used by the patient safely and effectively. |
|
| 2 | Pharmacology | Pharmacology is also one of the core branches of pharmacy. It deals with the uses, effects, and modes of drug action on biological systems. |
|
| 3 | Novel Drug Delivery System (NDDS) | The NDDS is an advanced drug delivery system that improves drug potency, and controls drug release to give a sustained therapeutic the effect, and provides more safety. NDDS is a unique combination of advanced techniques and new dosage forms; that will be superior activity-wise as compared to conventional dosage forms. |
|
| 4 | Targeted Drug Delivery System | Targeted drug delivery is a modern method of delivering drugs to the patients in a targeted sequence that increases the the concentration of the drug delivered to the targeted like organs, tissues, and cells. This turn improves the efficacy of treatment and reduces the side effects of the drug administration. |
|
| 5 | Pharmaceutical Nanotechnology | Pharmaceutical nanotechnology embraces applications of nanoscience to the pharmacy as nano-materials, and as devices like drug delivery, diagnostic, imaging, and biosensor. It has taken a boom in the last decade and is also set to revolutionize the pharmaceutical industry. |
|
| 6 | Nano-particles |
|
|
| 7 | In vitro evaluation |
In vitro means within the glass with standard procedure in a controlled environment outside of a living organism. |
|
| 8 | In vivo evaluation |
In vivo means within the living. It refers to experimentation using a whole, living organism as opposed to a partial/dead organism. Animal studies and clinical trials are the two forms of in vivo research. |
|
| 9 | Ex-vivo evaluation of plant extracts and formulations | Ex-vivo means Out of the living. In laboratory science, ex vivo evaluation refers to the experimentation or measurements done in or on tissue taken from a test organism within a simulated external environment under natural conditions. |
|
| 10 | Pro-drug design | A pro-drug is a medication compound. A pro-drug might use to improve how a medicine is absorbed, distributed, metabolized, and excreted. The drug is designed to improving in the bio-availability when a drug itself poorly absorbed from the gastrointestinal tract. |
|
| 11 | Pharmacokinetic studies | Pharmacokinetics is defined a branch of pharmacology that deals with the absorption, distribution, metabolism, and excretion of drugs and their relationship with the onset, duration, and intensity of the drug effect. |
|
| 12 | Pharmacodynamic studies | Pharmacodynamics is a branch of pharmacology. It deals mechanism action of the drugs and the relation between the drug concentration and its effect. This kind of study is directly related to the physical and chemical effects of the drug on the body, parasites, and microorganisms. |
|
| 13 | Pharmacogenomics | This is new combined filed of pharmacology and genomics to develop effective, safe medications and doses based drugs. |
|
| 14 | Pharmacoepidemiology | Pharmacoepidemiology is related to the therapeutic effect(s), risk, and use of drugs. |
|
| 15 | Physical pharmacy | It is that area of pharmacy that deals with the quantitative and theoretical principles of science as they apply to pharmacy practices. |
|
| 16 | Toxicology | Toxicology is another branch of pharmacology with a record of the adverse events of drugs on the body and the branch deals with various ways for the drug study like symptoms, mechanisms, treatment, and chemical based poison. |
|
| 17 | Chemotherapy | Chemotherapy refers to treating diseases by chemicals that kill the cells, especially those of microorganisms and neoplastic cells. It has classified into two divisions first is antibiotics, and second is antineoplastics. |
|
| 18 | Comparative pharmacology | It is that branch of pharmacology. It deals with comparison-based studies of one drug to another. The drug may belongs to the same group or another group. |
|
| 19 | Animal pharmacology | Animal pharmacology deals with the different properties of drugs in animals. The various animal is being utilised for testing like rabbits, mice guinea pigs, etc. The drug will give to the animals for study parameters (their behaviour, activities, vital signs, etc.) to be recorded. These are ones to evaluate the drug entity before testing it on humans. |
|
| 20 | Posology | Posology is the branch of pharmacology that deals with the dosage of drugs. |
Data Science Tools for Biopharmaceutical Professionals
Biopharmaceutical professionals will develop novel candidate molecules after acquiring the skill of data science tools through various courses, which will do by numerous organizations in offline and online modes. Nowadays, online mode is the most suitable way to acquire knowledge and skill through the certificate & degree courses and also take 100% free of cost certificate courses like Analytics Vidhya. You can choose your free certificate courses and start learning as per your requirement. The author has already experienced learning about data science tools through free online courses from the Analytics Vidhya website like ML, AI, DL & NPL.
Applications of ML and AIL to Biopharmaceutical industries
The emerging market for AI in drug development, valued at $700 million in 2018, is predicted to increase to more than 5 billion dollars in 2024. In continuation of the above, I would like to mention that new and novel drug development is costly (estimated to cost about $2.6 billion) and time-consuming (near about 10-20 years) affairs and the clinical trial success rate for new drugs hovers around 10% as the previously published article. The use of AIL in drug development is promising because it can potentially accelerate every stage of the R&D process while significantly reducing costs. For example, AI is used in the biopharma industry to identify new candidate drugs, which can be screened, validated, and optimized by AI-driven, integrated structural, target, and pathway-based data analysis. The generation of predictive in silico profiles of candidate drugs can reduce the time and cost of preclinical decision-making while increasing the likelihood of success for compounds selected for clinical trials. Further, the design of clinical trials to test novel drugs can benefit from predictive analytics, which can analyze clinical and genetic information to identify appropriate patient populations for trial inclusion to improve treatment outcomes.
It will also play a crucial role in other areas like Diagnostics, which is highly amenable to Artificial Intelligence (AI). AI-based analytics can sift through massive set of patient data to identify differences between healthy and unhealthy patients. For example, AI-based visual pattern recognition can apply to the diagnosis of patients in biomedical imaging, including x-ray, radiography, computed tomography (“CT”), magnetic resonance imaging (“MRI”), mammography, and ultrasound. Machine learning techniques (including deep learning) are currently 5–10% more accurate than the average physician; a gap expected to widen by eight false positives per image; scientist detects 92.4% of the tumors, relative to 82.7% by the previous best-automated approach. For comparison purposes, a human pathologist attempting an exhaustive search achieved 73.2% sensitivity”]. When applied to genomic datasets, AI algorithms can identify mutations with a significantly lower error rate, flagging them as pathogenic while revealing crucial information about the molecular pathways involved in cancer.
Based on earlier work, AI technology is applied successfully in preparing well-annotated data sets in the omics field (genomics, proteomics, transcriptomics, and metabolomics). AI can yield significant advances in personalized medicine, optimizing treatment decision-making for an individual patient and predicting treatment responses to candidate drugs through integrating patient health data (Genetic, epigenetic, and medical records).
Knowledge of biochemistry, structural biology, immunological principles, microbiology, and genomics) of the professionals has increased dramatically in recent years. There has been tremendous growth in data science, informatics, and artificial intelligence needed to handle this immense data flow. Bioinformatics is a unique field of combination of Biological science and data science, where we can apply computational tools to develop result-oriented applications only with a better understanding of both fields. Within these fields, many new databases and computational tools have created that increasingly drive immunology research, in a case drawing upon artificial intelligence and machine learning to predict complex immune system behaviors.
For example Prediction of B cell and T cell epitopes, we provide an overview of computational tools and artificial intelligence used for protein modeling, drug screening, and designing new vaccines against infectious agents. These tools can use with transform approaches to pandemic countermeasure development during the current COVID-19 pandemic.
Major Thrust Areas of the Biopharmaceutical Industry
Based on the available literature, the author has summarized four major thrust areas of the Biopharmaceutical industry in the current situation as described below:
Thrust areas 1 – To design and develop novel candidates for vaccines
Thrust areas 2 – To design and develop novel molecules of drugs
Thrust areas 3 – To improve processing for vaccine production
Thrust areas 4 – To monitor safety data of vaccine
In this article, the author will discuss only thrust areas 1 & 2 through examples as a significant contribution of data science tools in the biopharmaceutical industry.
Thrust areas 1: Potential of machine learning in improving the effectiveness of the vaccine
The author is describing with the help of an article titled SIMON: The machine learning platform paving the future of vaccine development written by Dr. Adriana Tomic from Oxford Vaccine Group (OVG) as an example. In this article, ML used the data science tool to solve the crucial problem of the flu vaccine.
Millions of people are suffering from the influenza virus infection, commonly known as flu. The flu kills up to 0.5 million people/ year worldwide are elderly and young children. Due to a high mutational rate, new flu strains appear regularly. Therefore there is a need to develop flu vaccines every year to protect against the circulating viral strains; otherwise, there is a risk of a global pandemic. Thus, it is critical to understand how seasonal vaccines protect against flu and are also helpful in preventing future pandemics.
The open-source platform was designed & developed with the help of a machine learning tool for applying the biomedical data by OVG. The developed platform is used for the experimental purpose of research by scientists (immunology and vaccinology), as described in figure 3.
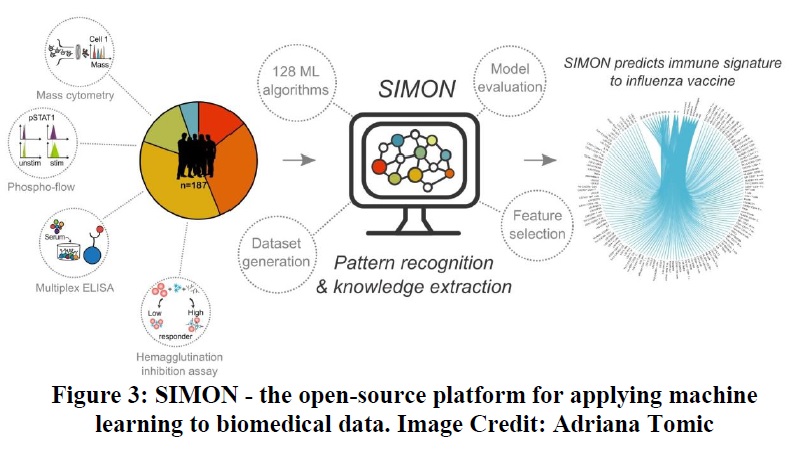
The software is open-source and free to download, as Tomic and the other developers believe it should be accessible to all to have the best impact. Vaccines for other diseases are also underway to examine for vaccination purposes. The Oxford Vaccine Group is trying to use meningococcal vaccines given to infants and vaccines against Salmonella typhi.
Thrust areas 2: Potential of the Deep learning branch of machine learning in drug discovery
The rapid growth in data science, generation of big data sets, and advancement in algorithms has increased the demand for data science tools (such as machine learning, artificial intelligence, etc.) in biopharmaceutical industries. On 30th May 2018, Nic Fleming reported in Nature journal that machine learning and other technologies are expecting to begin a hunt for new pharmaceuticals quicker, cheaper, and more effective, especially in drug discovery research. For understanding purposes, one article is described here as an example. One article published on Drug Discovery by using Deep Learning tool only with 10 Lines of Codes, and we will discuss this article as an example given below Figure 4:
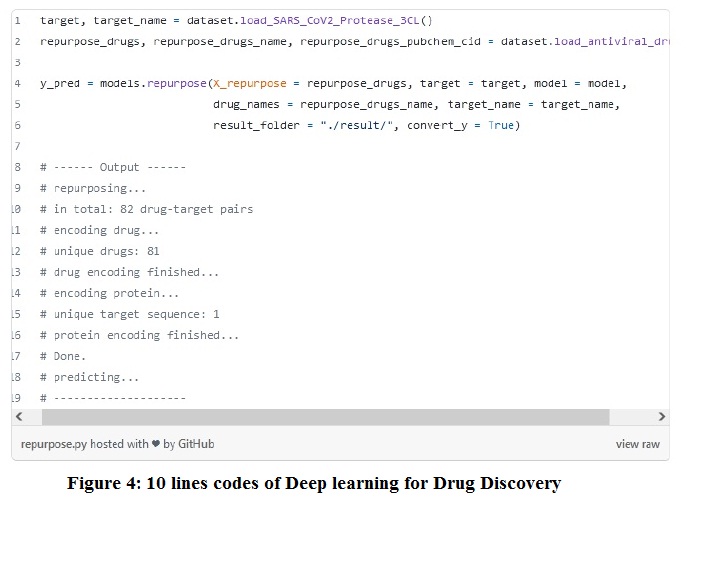
For example, a code-based case is presented in two easy steps for understanding purposes as an application.
Step 1: Re-purposing from a set of antiviral drugs for a COVID-19 target 3CL protease.
- Corresponding data in the data set is described in figure 4.
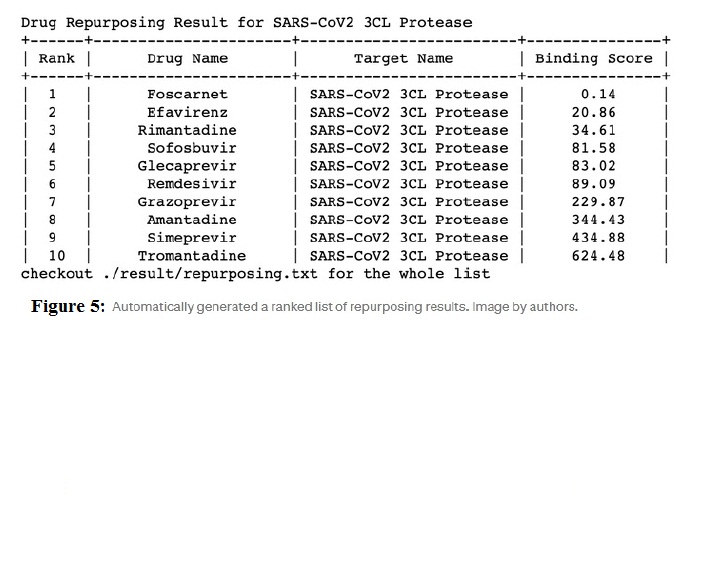
- Automatically generated list of the drug candidate in printout form as given in figure 5.
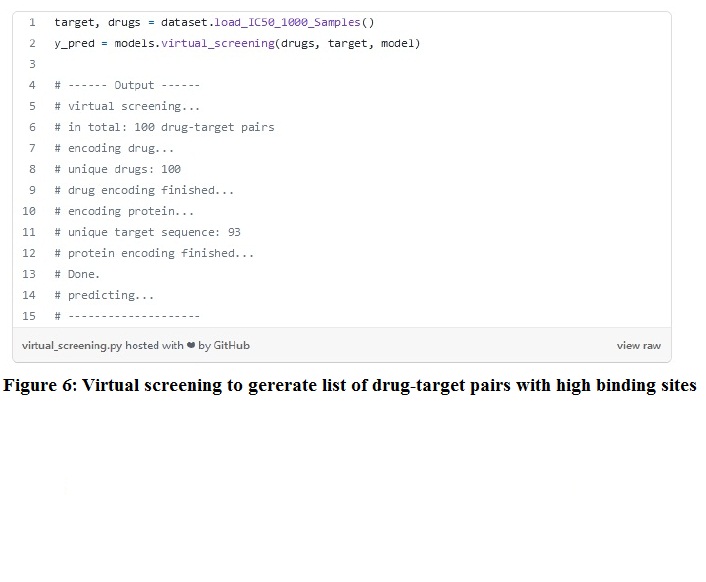
Step 2: Virtual screening on a sample of data from the BindingDB data set and then using the virtual_screening function to generate a list of drug-target pairs with high binding affinities. The corresponding data in the data set is described in figure 6. If no drug is selected as a screened drug, you can choose anyone from the default drug list. A similar-looking ranked list would generate.
Conclusion
Based on need, problem-solving & knowledge gaps, Biopharmaceutical and Data Science industries are growing and most demanding industries nowadays. Therefore present article will help to provide knowledge for tagging the thrush areas of biopharmaceuticals and encourage budding scientists and actively engaged scientists. Those are working in any field of the biopharmaceutical industry with knowledge of data science tools. At the end of this article, the author strongly suggests and concludes the following points:
– Biopharmaceutical professional to acquire knowledge of data science tools through various online and offline certificates, diplomas, nano-degree, graduate, and post-graduate courses.
– Based on the acquired knowledge, the professionals will select and start working on the thrust area.
– Author has already tagged four major thrust areas of the biopharmaceutical industry designing & develops novel vaccine candidates, designing & develops novel drug molecules, improving the involved procedure of vaccine production, and monitoring vaccine safety.
– Author has also encouraged the professionals to contribute to public health issues through this newly developed field.
I am happy if any reader will get motivated to start work in this field after reading this article, and I am always open to discussing the article content on my e.mail ([email protected]).
The media shown in this article is not owned by Analytics Vidhya and is used at the Author’s discretion.




